Samarium »
PDB 1a3c-5ktj »
2o5w »
Samarium in PDB 2o5w: Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate
Protein crystallography data
The structure of Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate, PDB code: 2o5w
was solved by
S.B.Gabelli,
M.A.Bianchet,
L.M.Amzel,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.97 / 2.60 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 124.416, 42.909, 108.328, 90.00, 115.19, 90.00 |
| R / Rfree (%) | 19.5 / 28.7 |
Other elements in 2o5w:
The structure of Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate also contains other interesting chemical elements:
| Sodium | (Na) | 2 atoms |
Samarium Binding Sites:
The binding sites of Samarium atom in the Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate
(pdb code 2o5w). This binding sites where shown within
5.0 Angstroms radius around Samarium atom.
In total 3 binding sites of Samarium where determined in the Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate, PDB code: 2o5w:
Jump to Samarium binding site number: 1; 2; 3;
In total 3 binding sites of Samarium where determined in the Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate, PDB code: 2o5w:
Jump to Samarium binding site number: 1; 2; 3;
Samarium binding site 1 out of 3 in 2o5w
Go back to
Samarium binding site 1 out
of 3 in the Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate
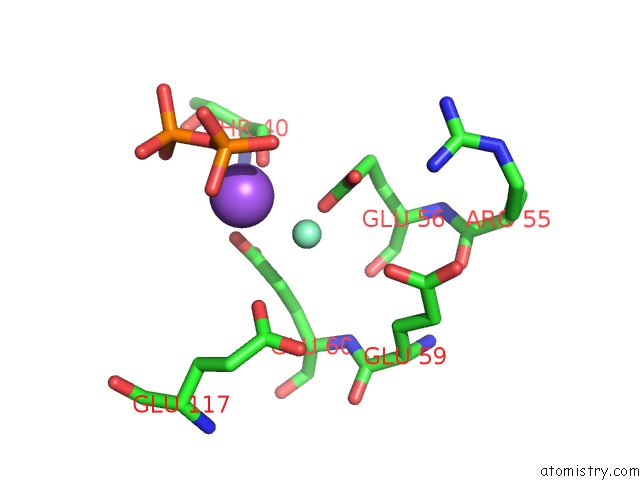
Mono view
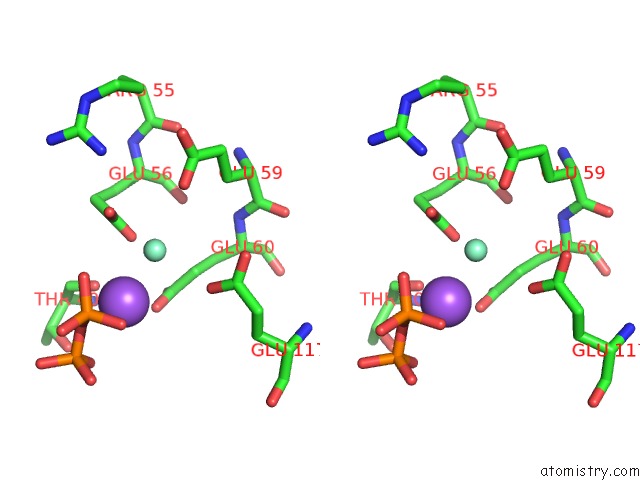
Stereo pair view

Mono view

Stereo pair view
A full contact list of Samarium with other atoms in the Sm binding
site number 1 of Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate within 5.0Å range:
|
Samarium binding site 2 out of 3 in 2o5w
Go back to
Samarium binding site 2 out
of 3 in the Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate
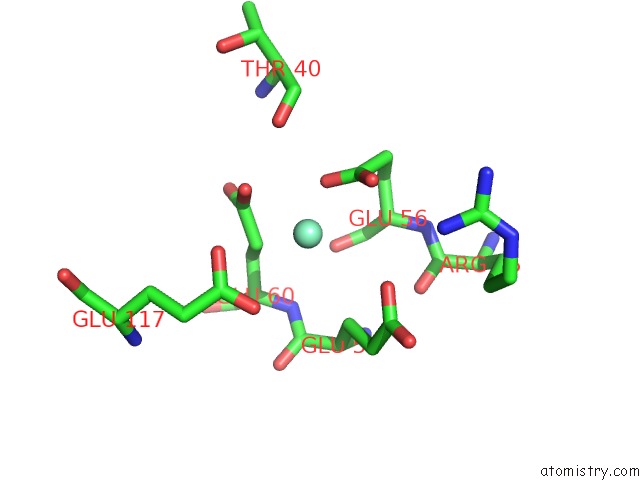
Mono view
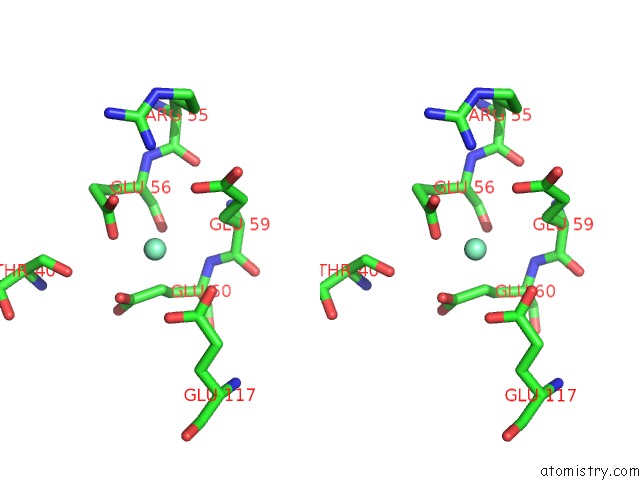
Stereo pair view

Mono view

Stereo pair view
A full contact list of Samarium with other atoms in the Sm binding
site number 2 of Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate within 5.0Å range:
|
Samarium binding site 3 out of 3 in 2o5w
Go back to
Samarium binding site 3 out
of 3 in the Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate
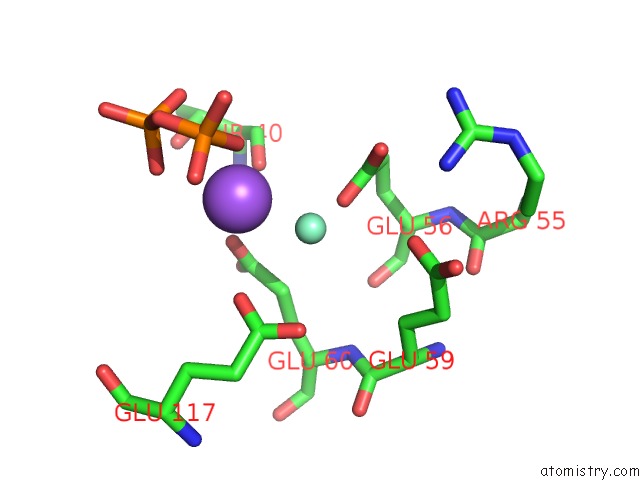
Mono view
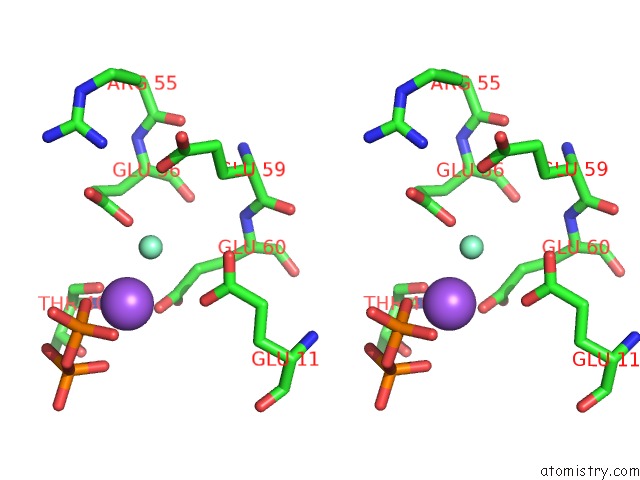
Stereo pair view

Mono view

Stereo pair view
A full contact list of Samarium with other atoms in the Sm binding
site number 3 of Structure of the E. Coli Dihydroneopterin Triphosphate Pyrophosphohydrolase in Complex with Sm+3 and Pyrophosphate within 5.0Å range:
|
Reference:
S.B.Gabelli,
M.A.Bianchet,
W.Xu,
C.A.Dunn,
Z.D.Niu,
L.M.Amzel,
M.J.Bessman.
Structure and Function of the E. Coli Dihydroneopterin Triphosphate Pyrophosphatase: A Nudix Enzyme Involved in Folate Biosynthesis. Structure V. 15 1014 2007.
ISSN: ISSN 0969-2126
PubMed: 17698004
DOI: 10.1016/J.STR.2007.06.018
Page generated: Thu Oct 10 13:50:32 2024
ISSN: ISSN 0969-2126
PubMed: 17698004
DOI: 10.1016/J.STR.2007.06.018
Last articles
Na in 6JZHNa in 6JY4
Na in 6JY3
Na in 6JUW
Na in 6JPR
Na in 6JSC
Na in 6JSG
Na in 6JNH
Na in 6JME
Na in 6JMV